A team of Sixth Form scientists were among the front-runners in a prestigious national Chemistry competition run by Cambridge University and a university in the Czech Republic.
 QE’s Chem Taj team were ranked fifth out of the 42 teams competing in the Cambridge Chemistry Race, many of them drawn from the country’s leading academic schools.
QE’s Chem Taj team were ranked fifth out of the 42 teams competing in the Cambridge Chemistry Race, many of them drawn from the country’s leading academic schools.
The five-strong team’s score of 113 points was actually identical to the scores recorded by the teams coming third and fourth, but, in line with the rules, they were given a lower ranking based on the nature of the questions they answered.
Congratulating the Year 13 team, Head of Chemistry Julia Lister said: “The boys adapted very well to the format of the competition, which had to be run entirely online because of the lockdown, keeping their heads under the pressure of competition as they raced to answer as many questions as they could within the two-hour time limit.”
The competition was originally scheduled to take place at the University of Cambridge’s Chemistry Department.
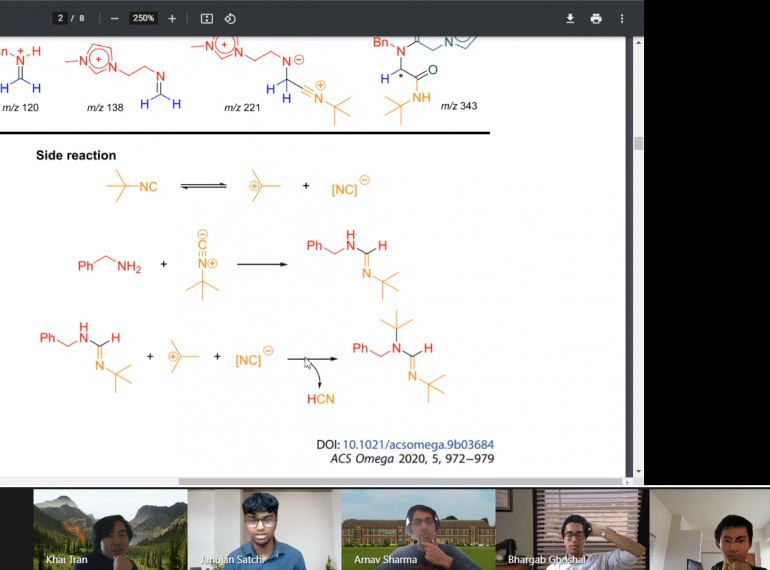
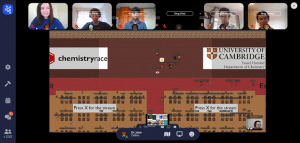
Instead, QE’s team members, Bhargab Ghoshal, Janujan Satchi, Arnav Sharma, James Tan and Khai Tran, worked from their own homes, communicating and completing the race through the Moodle online platform. The event’s opening and closing ceremonies were broadcast on YouTube, while the video-calling platform, Gather Town, was also used.
The boys are pictured, top, at work on a problem, and above right with Dr Lister during the competition.
James said: “Although I was disappointed the competition was not taking place in Cambridge as planned, I still enjoyed it and found it to be a welcome distraction during lockdown. In particular, I liked how the questions built upon our current A-level knowledge, but also required us to research into more advanced topics, such as hemiacetals and macrocycles.”
 Khai agreed, saying “it was interesting to apply the Chemistry we’ve learnt to topics beyond our specification”, while Janujan said that by working as a team, they were able to “apply different thought processes to the problem”.
Khai agreed, saying “it was interesting to apply the Chemistry we’ve learnt to topics beyond our specification”, while Janujan said that by working as a team, they were able to “apply different thought processes to the problem”.
Chemistry Race originated in 2015 as a Czech competition, called Chemiklání, at the University of Pardubice in the city of Pardubice, 96km east of Prague. It proved to be extremely popular among Czech pupils, and the scale of the competition rapidly grew every year.
In 2020, Adam Přáda, one of the organisers of the Czech competition and a Cambridge student, initiated a Cambridge branch of the competition, the Cambridge Chemistry Race.
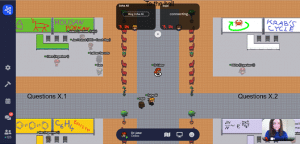
Once a team answers a question successfully, they are presented with a new one and a live leader-board is updated. Half-an-hour before the ‘race’ ends, the ranking is hidden; the final winners are announced at the closing ceremony.
The questions include easy riddles, tasks of A-level difficulty and more complex chemical problems. Competitors are allowed to use books or notes, since the questions mainly aim to test problem-solving skills and chemical understanding, rather than knowledge.
Questions asked in the competition included:
Q. Imagine that somebody loses 10 kg of fat over three months. 10 kg is quite a significant amount of matter; however, we don’t see pieces of meat falling off people who lose weight. How does the “fat” leave the body? Write down the formulae of the two main substances into which fat is broken down.
A. Carbon dioxide and water. One could figure this out using common sense, by realising that we exhale carbon dioxide all the time and that camels “store” water into fat.
Q. What is the highest possible mass percentage of hydrogen (H) for a neutral hydrocarbon? Give the answer as a percentage.
A. Maximizing the mass percentage of hydrogen can be done in two ways: by complete saturation (thus we consider alkanes) and by minimizing the number of carbon atoms. This is done, because for alkanes (empirical formula CnH2n+2) the addition of one carbon atom and two hydrogen atoms shifts the mass percentages in favour of carbon. We therefore seek the shortest alkane: methane. The mass percentage of hydrogen in methane is then 25.1 %.
And finally, this was identified as one of “this year’s more difficult problems” in the closing ceremony:
Q. How many atoms are there in 1.66×10-24 moles of argon.
A. Just one.
